notes college physics electricity thermodynamics optics waves circuits magnetism
f601fce @ 2022-12-20
8093 Words
Physics 2
Class Information
This second semester calculus-based introductory physics course is a follow-up to Physics 1061. The course focuses on developing algorithmic problem-solving skills and is intended as a preparation for advanced courses in physics as well as preparation for further study in upper division science and engineering. Topics include temperature, heat and the first law of thermodynamics, kinetic theory of gases, entropy and the second law of thermodynamics, electrical charges, the electric field, Gauss’s Law, electrostatic potential, capacitors and dielectrics, current, resistance, Kirchhoff’s laws, the magnetic field, Ampere’s Law, Faraday’s Law, inductance, geometrical optics, and interference and diffraction of light.
Textbook: Physics for Scientists and Engineers A Strategic Approach with Modern Physics (2016, Pearson) by Randall Knight
18: A Macroscopic Description of Matter
Solids, Gases, Liquids
- Temperature: Amount of thermal energy
- Ideal Gas: Used for modeling. Consists of tiny, hard spheres that collide but don’t interact with each other in any other way.
- Ideal Gas Law:
- Ideal Gas Law:
- Solids and Liquid are nearly incompressible, gases are compressible
- State Variables: When taken together, describe state of macroscopic system
- Thermal Equilibrium: When state variables are constant
Atoms and Moles
-
The Number of Particles is denoted by
-
Number Density: Number of atoms/molecules per cubic meter
- For a uniform system, the number density is the same whether you look at a portion or the whole system and is independent of the volume,
- For a uniform system, the number density is the same whether you look at a portion or the whole system and is independent of the volume,
-
Atomic Mass Number: number of protons + number of neutrons
-
Atomic Mass Unit (U): 12 U = Mass of Carbon-12
-
Molecular Mass: Sum of atomic masses for a molecule
-
Avogadro’s Number:
-
The number of moles of a substance is denoted by
-
Molar Mass
-
For a system of mass,
Temperature
Thermal Energy: Kinetic and potential energy as particles vibrate or move
- Linear relationship between
- All gases extrapolate to zero pressure at -273 C
- Absolute Zero: When all movement of particles ceases
Thermal Expansion
- Objects expand when heated
- For solids, where
- Solids expand linearly in all directions
- For a cube of length,
- The above derivation from the textbook isn’t very clear, so https://physics.stackexchange.com/a/386024
- Volume Expansion, where
- Water is weird between 4C and 0C (expands instead of contracting)
Phase Changes
- Phase Equilibrium: When more than one phase can coexist; two phases are in phase equilibrium along a phase boundary
- Slope of Solid-Liquid boundary layer differs between water and
- If you compress
- However, compressing ice along the boundary turns it into liquid water due to the negative slope of the boundary
- If you compress
- Critical Point: Where the liquid-gas boundary ends; No clear distinction between liquid and gas or phase changes exists here
- Triple Point: The one point at a specific temperature and pressure where all three phases are at equilibrium (all phases can coexist)
- The Kelvin scale used to be defined as the scale starting at 0 K and passing through 273.16 K (the triple point of water)
Ideal Gases
- Ideal Gases: Ignore weak attractions between each particle and treat each particle as “hard spheres” (elastic collisions); Treat all gases as consisting of just single particles
- Ideal gases fail to describe the correct behaviour for the following conditions:
- Density is low
- Temperature is well above the condensation point
- Graphing a PV vs. nT graph for any gas yields the same slope
- Slope = R = 8.31 J /mol K
- Boltzmann’s Constant:
- The Ideal Gas Law applies only when state variables are constant and not changing, but we assume the state variables are changing so slowly that the system is never far from equilibrium
- Quasi-static process: Process that is at thermal equilibrium at all times
- Quasi-static processes are reversible
- Isochoric Process: Constant volume process
- Vertical line for PV diagram
- Isobaric Process: Constant pressure process
- Horizontal line for PV diagram
- Isothermal Process: Constant temperature
- Hyperbola for PV diagram
19: Work, Heat, and the First Law of Thermodynamics
Energy Principle
- Mechanical Energy: Kinetic and potential energy
-
Isolated System:
-
There seems to be another way to transfer energy in a system since when heating a pot of water, there seems to be no external work done, yet the thermal energy increases.
-
Heat (Q): Another way to transfer energy into a system through thermal interactions
Work in Ideal-Gas Processes
-
Mechanical Interaction: System and environment interact via macroscopic pushes and pulls
-
Mechanical Equilibrium: No net force on system
-
Work is not a state variable, unlike thermal energy and mechanical energy
- Work is the amount of energy that moves between a system and environment (So never use
- Work is the amount of energy that moves between a system and environment (So never use
-
- The environment can do either positive work on the gas or negative work on the gas (which means the gas is actually doing work on the environment)
- Note: From now on
Isochoric Process
Isobaric Process
Isothermal Process
Work Depends on the Path
- Work done during an ideal-gas process depends on the path in the pV diagram (path here does not represent a physical path)
- Work is independent of the path for only work done by conservative forces (physical path)
Heat
- Work and heat are equivalent
- Thermal Interactions: No macroscopic interactions unlike mechanical interactions, used by heat to transfer energy
- Thermal Equilibrium: No temperature difference
- Heat is not a state variable, so
- Heat is not the only way to change temperature since work can also change the temperature of a system
First Law of Thermodynamics
- Assume
- First Law of Thermodynamics:
Isothermal Process
- Temperature doesn’t change, because heat and work are exchanged
Isochoric Process
Adiabatic Process
- No heat is transferred
- Temperature can still change
- Adiabatic curves are steeper than isotherms
Thermal Properties of Matter
- Specific Heat: Amount of energy needed to raise 1kg of a substance by a 1K
- For most liquids and solids, we heat the matter instead of doing work
- The heat of transformation (L) is the energy needed to make a substance undergo a phase change
Calorimetry
If there is sufficient insulation
The Specific Heats of Gases
Isothermal
Isochoric
Isobaric
Any Ideal Process
-
Any two processes that change the thermal energy by
-
For any ideal-gas process
-
Heat depends on the path on the pV diagram
-
Monatomic Gases:
-
Diatomic Gases:
-
For all ideal gases:
Adiabatic Process
- Image from wikipedia

- Temperature decreases during the adiabatic expansion
- Temperature increases during the adiabatic compression
Heat-Transfer Mechanisms
Conduction
- Thermal Conductivity: Represented by
- Heat transferred through direct physical contact
- Heat flows due to a difference in temperature
- Conduction results do to the collision of particles at the interface between the two materials
- Molecules from hotter region collide and transfer energy to those in an adjacent cooler region
Convection
- Transfer of thermal energy by moving fluids
- Air is a poor conductor of heat, but thermal energy is transferred easily due to convection
- No simple equation due to turbulence
Radiation
- Electromagnetic waves carry energy
- Emissivity (e): How effectively a surface radiates energy
- Stefan-Boltzmann Constant (
- Since objects emit and absorb radiation, the net amount of radiated power:
- Black body: Perfect absorber and emitter
20: The Micro/Macro Connection
Questions
We still have the following questions:
- Why does the ideal gas law work on every gas?
- Why is the molar specific heat (
- What is Temperature?
- Why does a gas have pressure?
Assumptions
- No intermolecular forces, so molecules only have kinetic energy, no potential energy
- Molecular motion is random (average speed is dependent on temperature)
- Collisions with the wall of the container are elastic
Molecular Speeds and Collisions
- There is a distribution of velocities for gas particles, not just one speed
- Pressure and temperature are based on the average of these speeds
Mean Free Path
- Mean Free Path (
- If a molecule has
- If a molecule has
- Two molecules collide if the distance between their centers is less than
- The number of collisions is equal to the number of molecules in a cylindrical volume of length
- The above derivation assumed one particle was colliding with a stationary target particle. If we don’t assume this, we get the following:
Pressure in a Gas
- Pressure comes from force over area. Force comes from change in momentum of a particle
On average, half of the particles collide with the wall during the
The Root-Mean-Square Speed
Temperature
Temperature is a measure of the average translational kinetic energy.
The following two equations relates macroscopic state variables (
We can assume collisions are elastic because if the collisions were inelastic, then the temperature of the gas would continue to decrease due to the loss of kinetic energy. This doesn’t happen in real life, so we can assume that collisions are elastic.
Thermal Energy and Specific Heat
Monatomic Gases
Atoms have no molecular bonds in an ideal gas so
Equipartition Theorem
- In addition to kinetic energy, non-monatomic gases can have the ofllowing forms of energy
- Kinetic and potential energy associated with the vibrations from the spring like bond between molecules
- Rotational Kinetic energy
- Degrees of Freedom: The number of independent modes of energy storage
- Monatomic have 3 degrees of freedom since there are 3 different types of translational kinetic energy along x, y, and z
- Equipartition Theorem: The thermal energy is distributed evenly among all the different possible types of degrees of freedom. Each degree has the following energy:
- Monatomic had 3 degrees so thermal energy was
- Monatomic had 3 degrees so thermal energy was
- Vibration: For diatomic 2 degrees of freedom (one for each atom)
- Rotational: For diatomic only 2 degrees (since rotation along one axis has no rotational kinetic energy)
Solids
- 6 degrees of freedom
- 3 translational kinetic and 3 vibrational (potential)
- 3 translational kinetic and 3 vibrational (potential)
Diatomic Molecules
- 8 total degrees of freedom but only 5 are available at room temperature due to quantum effects
Thermal Interactions and Heat
- Heat is the energy transferred via collisions
- When thermal equilibrium is reached the following is true
Second Law of Thermodynamics
-
Equilibrium is the most probable state
-
Entropy: Measures the probability that a macroscopic state will occur spontaneously or the measure of disorder
-
Reversible microscopic events lead to irreversible macroscopic behavior since some macroscopic states are more probable
-
Second Law of Thermodynamics: The entropy of a system never decreases
- Heat always travels from hot to cold
21: Heat Engines and Refrigerators
- Practical devices transform heat into work
- All devices must obey two two laws of thermodynamics
- Energy is conserved
- Most macroscopic processes are irreversible. Heat energy is transferred spontaneously from a hooter system to a cold system but never the other way around
- Energy is conserved
Questions
- What are the limitations imposed by the above laws on these practical devices
- How do these devices transform heat into work?
Heat into Work
-
So far we’ve defined
-
Energy is transferred into the system as heat to do work or stored within the system as increased thermal energy
-
Heat Reservoir: An object that is so large that its temperature does not change when heat is transferred between the system and reservoir
-
-
-
By definition
-
Converting heat to work can be done with thermal expansion, but the system is at a different state. A heat engine must be a closed cycle.
Heat Engine
- Clockwise PV diagram
- Extract heat,
- Do useful work
- Exhausts heat energy (
Sterling Engine
- Two isotherms and two isochoric processes in one cycle
- Heat transfers occur in all four processes
Thermal Efficiency
- The purpose of heat engines is to transform as much of the heat absorbed
- Thermal Efficiency is denoted by
- Actual engines have
Ideal-Gas Heat Engines
- An ideal gas heat engine can be represented by a clock-wise loop
- The net work is the area inside the loop, not the area under the loop
Refrigerator
Opposite of heat engine (Counter clockwise PV diagram)
- A Refrigerator transfers heat out of its cooler interior to its warmer surroundings
- Refrigerators don’t violate the 2nd Law since you have to “pay” to have heat flow from
- Requires
- Requires
- In any closed-cycle refrigerator, all state variables return to their initial values once every cycle
- Over one cycle:
- 1st Law
Efficiency
Brayton Cycle
- Ideal-Gas refrigerator that uses adiabatic compression and adiabatic expansion to quickly heat and cool system, respectively
- Two adiabatic processes
- Two isobaric processes
- Reverse Brayton cycle is for refrigerators and regular is for heat engines
No Perfect Heat Engine
- No perfect heat engine with
- A Perfect Heat Engine means
- This combo violates the second law. Thus all heat engines MUST output some
Limits of Efficiency
-
Question: Is there a maximum efficiency or max COP (Coefficient of performance) for a device operating between
-
Answer: Yes
Carnot Engine
- Carnot Engine: Perfectly reversible engine
- Carnot Cycle
- Two Adiabatic processes (
- Two isothermal processes (
- Two Adiabatic processes (
Maximum Efficiency
- Second Law (informal statement): No heat engine operating between energy reservoirs at
- Second Law (informal statement): No refrigerator operating between energy reservoirs at
22: Electric Charge and Force
- Goal: Learn to calculate and use the electric field
- Questions
- What is Coulomb’s Law?
- How to determine the electric force on a point charge?
- What is an electric field?
- What is the electric fields of a point charge?
- How to calculate the electric field of discrete charge distribution?
Electric Charges
- Proton and electron
Electric Forces
- Coulomb’s Law: A force occurs for point charges that are separated by a distance
- For two positively/negatively charged particles, they experience a repulsive force of the magnitude.
- Permittivity constant
- Charge of one electron or proton is
Forces on Point Charges
- Add up vectors
Use coulomb’s Law to get magnitudes
The Field Model
23: The Electric Field
-
Electric field created by charge
-
The Electric Model
-
Long range of interaction a distance
-
Unit of Electric field are
-
In the Field Diagram for protons, the field lines points a away from from source. For an electron, the source acts as a sink.
The Dipole: An Important Charge Distribution
- Electric dipole: Consists of two point charges of equal magnitude but opposite signs, held a short distance apart
- Many molecules can be modeled as dipoles (e.g. water)
For a point lying on the axis of the dipole
For a point perpendicular to the dipole
Where
Continuous Charge Distributions
Question: What if the charge is continuous (not discrete)?
For macroscopic charged objects, like rods or disks, we assume the charge has a continuous distribution.
- Divide the objects into small point charge-like pieces
- The summation of fields of an infinite number of infinitesimally small pieces means integration.
An Infinite Line of Charge
- A straight infinite line of charge coincides with the x-axis and the line carries uniform charge with the charge density
- The x-component of two equidistant points on the line will cancel out
- Only the y-component adds up
with
- Notice that the electric field is proportional to
Ring of Charge
- A ring of radius
- Only x-components add up
- At the center of the ring, the net electric field is 0
Disk of Charge
where the z-axis passes through the center of the disk and the point lies on the z-axis
Infinite Plane of Charge
-
An infinite plane is just a disk with radius of infinity
-
The electric field is the same at any point in space regardless of how “far” away a point is from the plane, since the plane is infinite
-
Sphere of Charge
Parallel-Plate Capacitor
- One plane is positively charged and one plane is negatively charged
- All charges are on one surface of hte plane because opposite charges attract
- For inside the capacitor
- A uniform electric field exists inside a parallel-plate capacitor
25: The Electric Potential
Potential from the Electric Field
-
Like gravity, the force exerted on a charge
-
Work can be represented by the change of electric potential energy
-
-
Potential Difference:
- Must define a reference point (V(A) = 0)
Potential Difference :Capacitor
A plate is equipotent
-
Define reference point
-
Choose path as straight line
-
Notice that since
Insert Diagram -
The electric potential decreases in the direction of the electric field
-
When U increases, K decreases, and vice versa
-
Positive charge: “downhill” means U goes down and K goes up
Electric Potential of a Point Charge
Insert Diagram
- Easier to use a path parallel to
- Notice that the second part of the path (the curve) is always perpendicular to the electric field. This means the contribution is
Electric Potential of a Charged Sphere
- Using Gauss’s Law, we know that outside the sphere, the field is the same as a point charge
If the potential at the surface
Electric Potential for Multiple Charges
- You can add electric potentials
Electric Potential for Ring
%
26: Potential and Field
Capacitors
- If two capacitors
- The potential difference across two capacitors,
Capacitance in Terms of Distance and Area
For a parallel-plate capacitor
27: Current and Resistance
Current
- Weakly bound valence in metals
- Normally electrons are moving randomly with no net flow of charge
- An electric field causes a slow drift at speed
-
Very small value
-
-
-
Most metals contribute 1 valence electron to the sea of electrons per atom
-
The number of electrons
Creating a Current
- When charges are moving, there is a non-zero electric field causing electrons to move and current to flow
Current is defined as the direction positive charges flow, so current is opposite the direction electrons flow
Conservation of Current and the Junction Rule
Law 1
Due to conservation of charge, the current must be the same at all cross-sections along a current-carrying wire
Law 2 (Kirchhoff’s Junction Law)
For a junction, the law of conservation of current requires that the sum of the current in the branches
Conductivity and Resistivity
- Assume
- Average Time between Collisions/Electron Lifetime/Scattering Time:
- Current is caused by an electric field
- Current density depends on the strength of the electric field
- Current density depends on the material’s conductivity
When
Superconductivity
- Resistance is actually zero
- Charge moves without an electric field
Ohm’s Law
If we assume the electric field is uniform:
Remember
If we assume
28: Fundamentals of Circuits
Kirchoff’s Loop Law
For a loop (any path that starts and ends at the same point), the law of conservation of energy requires
Note: this can be true only if at least one
Let us define
- From negative to positive for a battery:
- From positive to negative for a battery:
- From positive to negative for a resistor:
Energy and Power
The rate of transfer of thermal energy through a resistor
29: Magnetism
-
Magnetic fields are similar to electric field, except that magnetic fields can only exist as dipoles
-
The force on a north pole is parallel to
Magnetic Field of a Moving Charge
- Biot-Savart Law
- The above is analogous to Coulomb’s Law
Superposition of Magnetic Fields
Add magnetic fields vectorally, the
Magnetic Field of a Long Straight Wire
where
Infinite Wire
N -Turn Current Loop
Magnetic Field of a Current Loop
On axis magnetic field (axis through center of loop):
For large distances
Solenoid
Magnetic Dipole Moment
A current loop is a magnetic dipole.
Magnetic Dipole is always perpendicular to the area of the loop (from south to north pole)
direction above is on the axis of the magnetic dipole
Cyclotron
Cyclotron frequency (1/T)
Cyclotron frequency does not depend on the speed of the particle!!!!
When the particle has a component of
- Perpendicular component is responsible for circular motion
- The parallel component is responsible for
Spectrometer: Charged Particle in B
- Positive ions of charge
- The particles follow a Clockwise (If
Find
Magnetic Force on a Current
Consider a segment of length
Note:
Magnetic Force Between Parallel Wires
If two parallel wires have currents that both go up, they will attract.
The wire on the left creates a magnetic field that goes into the page on the right wire. So the right wire is pulled to the left. The right wire creates a magnetic field out of the page on the left wire. Thus the left wire has a force to the right.
Forces and Torques on Loops
We know a current loop is a magnetic dipole
Consider a
Applicable to any loop of any shape
30: Electromagnetic Induction
Induced Current
Question: If electric currents produce magnetic fields, is the reverse possible?
Magnetic Flux
Magnetic flux of
Note that the
When
1 weber = 1 Wb = 1
Faraday’s Law
If we slide a conducting wire along a U-shaped conducting rail:
Lenz’s Law
- Question: Which direction is the induced current
- An induced current always moves in the direction that its magnetic field opposes the original change of flux
The magnetic field
- in the opposite direction to the original
- in the same direction as the original
- is zero if the flux is not changing
Induced Electric Fields
- There must be an induced electric field tangent to the loop at all points
- EMF = the work per unit charge done as a charge goes around a closed loop
For an induced electric field that is produced by a changing magnetic field producing “pinwheel” pattern of induced electric fields
where
And the area of the magnetic flux is only the area formed by the real or imaginary closed curve of integration (loop)
Solenoid Induced Electric Field
Generalization of Faraday’s Law
- Direction of electric field follows Lenz’s Law
Mutual Inductance
Suppose there are two coils: Coil 1 has a changing current
Inductance is a property of Coil 2, denoted by
Self-Inductance
A changing current in a coil wil also induce an EMF
Example: For solenoid of length
Magnetic Energy in an Inductor
- Energy can be stored in an electric field of a capacitor
- Similarly, energy can be stored in a magnetic field of an inductor
Magnetic Energy Density
- For an ideal solenoid
Energy per unit volume, Magnetic Energy Density
Although the energy density was derived via a solenoid, these equations are universal.
Generators
Transformers
31: Electromagnetic Fields and Waves
Maxwell’s Law
- Charged particles create an electric field
- There are no isolated magnetic poles
- An electric field can also be created by a changing magnetic field
- Currents (or changing electric flux) create a magnetic field
Maxwell’s Correction to Ampere’s Law
-
Ampere’s Law only works for steady currents
-
An example of when ampere’s law fails is with a capacitor
-
A changing electric field flux can also create magnetic field as well
-
The electric field is
-
The electric flux (through
-
The rate the flux changes (through
-
Maxwell added a correction term to Ampere’s Law called the displacement current
Ampere-Maxwell (AM) Law
- The A-M law states that either a changing electric flux or electric current through a closed loop creates a magnetic flux around the loop
- A magnetic field can be created either by an electric current or by a changing electric field
Maxwell’s Equations in Vacuum (Special Case)
- In a vacuum (empty space) there is no electric charge (
Gauss’s Law (
Gauss’s Law (
Faraday
Ampere
- Complete symmetry between magnetic field and electric field
- The only source of either field is the change in time of the other field
Plane Waves
- Simplest solution of Maxwell’s equations is the plane wave
- A plane wave consists of E&M fields that vary in space ONLY in the direction of the wave propagation
- The plane is normal to the direction of propagation
- The electric field and magnetic field is the same everywhere on the plane
Let the
Properties of Plane EM Waves
-
Wave Number:
-
Angular Frequency:
-
Velocity
-
The plane wave only satisfies Maxwell’s equations in a vacuum if the wave speed is given by
Results
- Light is an EM wave
- Optics can be viewed as a branch of E&M
Intensity and Power
The Poynting vector,
The above relates the intensity of an EM wave to the amplitude of the wave’s electric field
Radiation Pressure
where
where
Polarization
Electric field vector and Poynting vector define the plane of polarization
Malus’s Law
33: Ray Optics
Ray Model of Light
- Light travels in straight lines
- Rays can cross without disturbing each other
- Rays travel forever until they interact with matter
- Objects are sources of light rays
Law of Reflection
- The incident ray and reflected ray are in the same plane normal to the surface
- The angle of reflection equals the angle of incidence
Refraction
Snell’s Law
- If
- Bending occurs because the wavelength
Mirrors
-
Flat: Virtual image only
-
Parabolic: All rays parallel to mirror axis converge to single focal point
-
Spherical Mirror: Approximates parabolic mirror close to the mirror
-
Focal Point (F): Any incoming parallel rays will be reflected towards this focal point
-
Center Point (C): The distance from this point to the mirror is the same in any direction
-
Real Image: Where two (or more) light rays converge
-
Virtual Image: An image formed when diverging rays appear to meet at a point “behind” the mirror
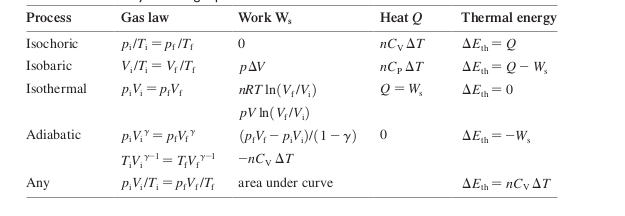
Concave Mirror
- Concave mirrors can create either real or virtual mirrors
- If
- If
- If
- Note that for a flat mirror, this is the case. The reflection is virtual and the focal pint is infinitely away from the mirror
- If
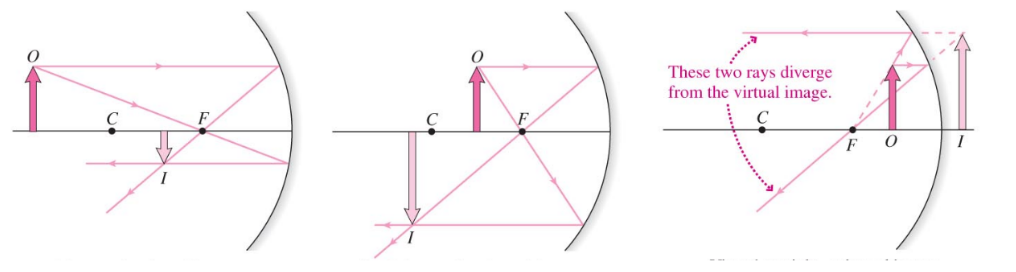
Convex Mirror
- Incoming rays reflects on the outside of its spherical curvature, causing light to diverge
- Features
- The focal point is behind the mirror (
- The image is virtual, upright, and reduced in size
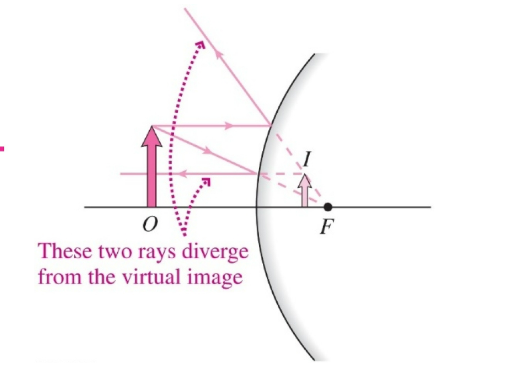
Mirror and Magnification equations
- If the image, focal point, or center of curvature is on the reflecting side of the mirror, the corresponding distance (
Images with Lenses
- Converging Lens which bring incoming parallel rays to focus
- Center thickeness is greater than edge thickness
- Diverging Lens: Bend parallel rays so they appear to diverge from a focus
-
Center thickeness is less than edge thickness
-
Thin Lens: Thickness is small compared to the curvature of radii of its two surfaces
-
Converging Lenses
- Double convex
- Planoconvex
- Convex Meniscus
-
Diverging Lenses
- Double concave
- Planoconcave
- Concave Meniscus
-
3 types of principal light rays
- parallel to the principal axis
- pass through the near focal point
- directed at the center of the lens
Converging Lens
- Can produce real and virtual images that are either enlarged or reduced
- 3 distinct cases
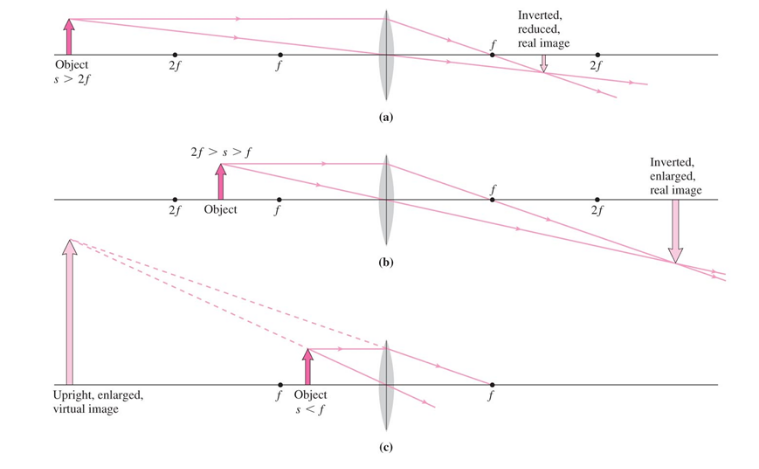
Diverging Lens
- Virtual image that is upright and reduced in size
- Virtual image is on the same size as the original object
Thin Lens Equation
Magnification Equation
Lens Sign Conventions
- Focal length is positive for converging lenses and negative for diverging lenses
- The object distance
- The image distance
- The height of the image
32: Wave Optics
Light can be viewed in two ways
- As a ray (see the last chapter)
- As a wave (this chapter)
The Wave Nature of Light can be seen through
- Double Slit Experiment
- Single Slit Experiment
- Diffraction
What is Light?
- Light is an electromagnetic wave
- Shares some similarity to water waves
- Water also experiences diffraction (spreading out of waves)
- The reason why light doesn’t experience diffraction in large openings is because light’s wavelength is quite small but most openings are quite large
The wave model
- When light passes through openings smaller than
The Ray Model - When light passes through openings larger than
Double Slit Experiment
Take a pair of slits, each
Two separate light waves overlap and interfere, resulting in a pattern of light and dark waves (similar to throwing two stones in water).
A Brief Review of Interference
Constructive Interference: A full-wavelength path difference results in constructive interference.
Destructive Interference: Crest meets trough, means waves cancel. Half-Wavelength path difference leads to destructive interference.
Bright Fringes (Constructive Interference)
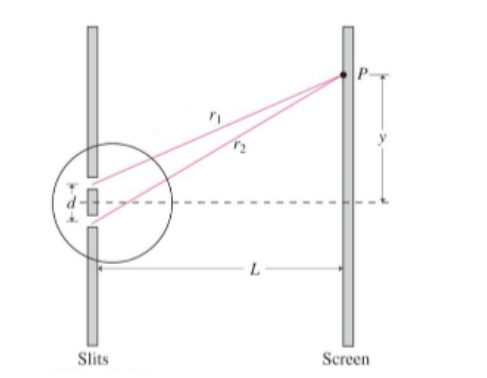
Note that
To find the actual
Dark Fringe
Fringe spacing is
Diffraction Grating
Diffraction Grating (DG): A system with many closely spaced slits
- d: the distance between narrow slits
- N: The number of lines per meter printed on a DG
Bright and narrow fringes follows the same rules:
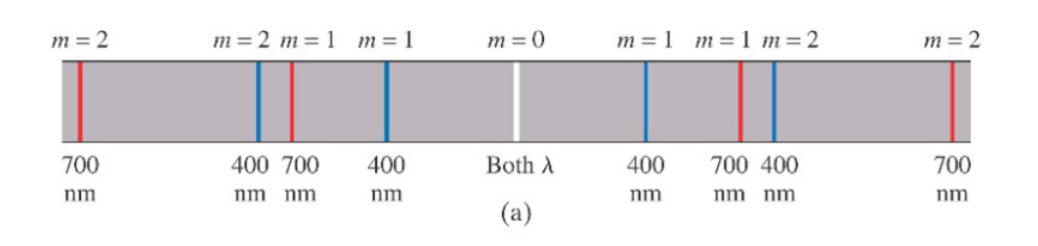
Consider what happens when white light passes through a diffraction grating.
For all frequencies, the path differences,
Single Slit Diffraction
- If we send a beam of monochromatic light through a single narrow slit, with viewing screen at distance
- Unlike with the double slit, there is one large, broad central maximum
- Perfect Destructive Interference- Minima:
- Width of Single-Slit Central Maximum
Circular-Aperture Diffraction
-
Q: What if the rectangular slit is replaced with a hole of diameter
-
A: The diffraction pattern of monochromatic light appears as a central circular max with second bright fringes ringed around
-
Angle of first min
-
Width of central maximum